こんな人におすすめの記事です。
- ChatGPTに論文執筆サポートをして欲しい
- ChatGPTが流行ってるみたいだけど、どうやって使えばいいのか分からない
こんなお悩みを解決します。
この記事では国立大学と外資系企業で研究者として働く私が、大学・企業の双方の経験を元に「大学からの企業転職でリサーチすべきポイント」を分かりやすくお伝えします。
最初は私も「AIなんかに論文かけるわけねーだろ」と思ってたんですが、実際に自分でChatGPTをいじってあまりのクオリティの高さに目から鱗が落ちました
本ブログは、私個人の責任で執筆され、所属する組織の見解を代表する物ではありません
ChatGPTを開こう
まずこちらのリンクに飛び、左下の「TryChatGPT」をクリックしてChatGPTの画面を開きましょう。
すると登録画面になりますので、Googleアカウントで登録すれば準備完了です。
あとは画面の「Send a message」に好きなオーダーを出してやればChatGPTさんが動き始めます。
ちなみに、ChatGPTにはいくつかバージョンがあり、私は有料版を使っているのでModel 4.0です。無料版はModel 3.5ですが、とりあえずお試しで無料版を使い、具合が良ければ有料版に移行すれば良いと思います。
なお、本記事で紹介するChatGPTを活用した論文執筆法ですが、私自身は”AI遊び”の一環として行っております。
可能な限りクオリティの高いアウトプットが出るようにプロンプトの推敲をいこないましたが、ChatGPTには正確性、著作権など科学的・倫理的に解決すべき課題があり、筆者は「ChatGPTに論文を書かせて科学誌に投稿しよう」という行為には現時点で賛成しておりません。あくまでも補助ツールとしてのみChatGPTを使うべきと考えております。
ChatGPTに論文を書く準備をさせよう

以下のプロンプトを入力します。
I want you to act as a researcher who draft a scientific paper. You will develop an scientifically relevant, impactful, and sophisticated scientific paper. I will provide you further detail prompts for each section of the study. Please say yes if you understand

ChatGPTでタイトルを書こう
以下のプロンプトを入力します。
OK, let’s start.
First section is the Title. You will develop an attractive and scientifically relevant title. I will provide you with the study objective and study design that can be used to develop the title.
Please note the following points.1. The expected role of the title is to tell the core concept of the paper and to attract readers
2. Do not use metaphors or vague expressions; use only scientifically rigorous words
3. Make the title precise and concise
4. Include information on the study design
5. Suggest 10 options
6. Include the pros and cons of each title
7. Make your suggestions as diverse as possible
8. Suggest in table format with one row for each title
If you understand, please suggest titles based on the following study objective and study design.
[Objective] To describe the treatment pattern for anti-dementia drugs in Japan using nationwide claims data
[Study design] Observational study
なお、黄色ハイライトは皆さん自身が作成したタイトルおよび研究デザインを入力してください。
すると、以下のようなアウトプットが返ってきました。

このうちお好みのものを選んであげましょう。
もし気にいるものがなければ、さらに修正指示を出してあげればOKです。
OK, I’d like to select number 4 for the title
「Excellent choice」と気分を上げてくれます。

ChatGPTでイントロを書こう
以下のプロンプトを入力します。
Next is the Introduction.
You will develop an attractive and scientifically relevant Introduction section of a scientific paper. I will further provide you the structure of the Introduction and useful information that can be used to develop the Introduction. However, you do not need to persist on these inputs if you have other attractive suggestions. The most important aspect is to emphasize the importance of the study, which can attract reviewers and readers to your paper. Also, please note that all your descriptions should be based on strong scientific evidence. Please do include no lies and delusions.
Please respond “Yes” if you understand my request.
忠実なるしもべよ!

そしたらイントロの大構造を伝え、箇条書きで小構造をセクションごとに提案をしてもらいます。
黄色ハイライトは皆さんの望む内容にリライトしても大丈夫です。
OK, Here are the inputs.
[High-level structure of the Introduction]
1. Background
2. What is known
3. What is unknown
4. Objective
Please suggest the detailed structure of the Introduction in bullet points. Each section of the Introduction should be consisted of at least three points.
そうすると45秒くらいで以下が出てきます、天才か…
しっくりこなければ再びここで修正指示を出しましょう。

私もChatGPTを使う前は「イントロダクションはアートや!AIなんぞに魅力的なものが書けるかい!」と思っていたんですが…
私ならこう書くかなーとか思ってたのと同じような提案がきて、「私の存在意義とは…」と落ち込んだよ。
さて、そうしたらいよいよイントロの本文執筆です。
以下のプロンプトを入力。黄色はご自由に。
Great job!
Please draft the Introduction based on the proposed structure taking care of the following points.
1. Make it precise and concise
2. Introduce concrete numbers in relevant sentences
3. Add references in number and provide the reference list at the end of the Introduction
4. Use more than 500 words but less than 1,000 words
5. Emphasize the importance and uniqueness of the study in the What is unknown or Objective section understandably, even for those who don’t have background knowledge in this area in terms of the study population, country, time, exposure, outcome, or data source, if applicable
If you understand, please start developing the Introductions.
すると1分程度でアウトプットが。



いやー、すごいですね。。。
ただ、極めて重要な注意点があります。
ChatGPTは事前指定がないと勝手に引用文献や数字を出してきますが、これは大嘘の可能性があるので信用しては行けません。
今回は「AI遊び」としてChatGPTをいじることが目的なので上記のやり方をしていますが、真剣にイントロ執筆にChatGPTを使うなら私は以下のやり方を推奨しています。
- 自分でひたすら文献を読み漁る(文献の検索式をChatGPTに提案してもらっても良い)
- そこからイントロの小構造を考え、ChatGPTにインプットする
- さらに、小構造のそれぞれのセクションを支えるエビデンスを先行研究から抜粋し、ChatGPTに食べさせる
- それを基にイントロの本文をドラフトするような指示を出す
つまり、構造や材料はこちらで考え、ChatGPTはあくまでも最後のドラフトの補助のみに使うという方法ですね。
実際にやってみた感じ、3で先行研究の抜粋&引用元のセットを50個くらい食べさせたくらいで嘘のないイントロを書いてくれるようになったよ
ちなみに完全に順序が逆ですが、提案されたイントロダクションに関連した文献を読んで知識を深めたければ、こんな感じで聞けば検索式を提案してくれます(黄色はご自由に)。
Great job! To enrich my knowledge, would you develop the search term in PubMed to search papers related to the proposed Introduction? Please note using MeSH term.

イントロを修正したければ、こんな感じで指示を出せます。
1. Please expand/simplify the description about ~ (ここを細かく・シンプルに)
2. Please add concrete numbers in the description about ~ (具体的に数字で)
3. Please further emphasize the importance of the study (研究の重要性を強調して)
ChatGPTの真価は単発のアウトプットではなく、修正指示に対する感度の高さだと思っています。
「思ったアウトプットがでない」理由はChatGPTの性能ではなく、プロンプトや修正指示側に原因がある可能性があるので、いろんな角度からインプットを検討しましょう。
例えば、上で提案されたイントロでは日本にフォーカスする理由の説明が弱いなと思ったので、試しに次のような修正指示を出しました。
Great job!
However, the suggested Introduction lacks a rationale for why this study focuses on Japan. Please add that rationale with relevant evidence.
すると、こんなに雑なインプットにも関わらず即座にBackgroundにて修正を反映してくれます。

ChatGPTでメソッドを書こう
お次はメソッドです。
ここはメソッドのセクションごとにさらに細かい指示をだせば精度がさらに急上昇しそうですが、今回はトライアルなのでシンプルなインプットに止めました。
Next is the Method.
You will develop an scientifically relevant method section in a scientific paper. I will provide you brief summary of the study design. Please say yes if you understand.
忠実でなにより!

そしたらメソッドの構造をインプットしましょう(黄色はご自由に)
OK, here are the inputs.
[Brief summary of the study design]
Study design: Observational cohort study
Setting: Japan
Data source: JMDC data (insurer-based health claims database)
Population: Patients with dementia
Outcome: Treatment pattern of antidementia drugs
Analyses: Descriptive analysesPlease start developing the method section of the paper.
メソッドの各セクションに最低限必要な情報だけをインプットしています。
するとこう返ってきました。


かなりシンプルで、これでは論文ではなくStudy Conceptです。
では、以下のようにさらに細かい指示を付け加えてやるとどうでしょうか?
OK, here are the inputs.
[Brief summary of the study design]
Study design: Observational cohort study
Setting: Japan
Data source: JMDC data (insurer-based health claims database)
Population: Patients with dementia
Outcome: Treatment pattern of antidementia drugs
Analyses: Descriptive analysesPlease taking care of the following points:
1. Expand the description of JMDC
2. Set a more specific definition of dementia by combining other criteria such as diagnostic tests or medical procedure
3. Add as many details as possible in the Outcome Measures and Statistical Analysis section
4. The method section should include all necessary details, eliminating room for misunderstandingPlease start developing the method section of the paper.
するとこう、各セクションが数行分だけ細かくなっています。


まだちょっともの足りません。
引き続き修正指示を与えましょう。
Great job, but it’s still lacking details.
Please revise it taking care of the following points:
1. Add further details of JMDC database
2. Add further details in the Outcome Measures section to remove the misinterpretation
3. Consider sensitivity analyses, additional analyses, and subgroup analyses to assess the robustness of the study design
するとこう(雑なインプットでごめんねChatGPT)。


確かにData SourceのJMDCの説明が詳しくなり、またStatistical Analysesに解析が追加されています。
でも、Outcome Measuresはあんまり詳しくなってませんね、追加で指示してみましょう。
OK, but the Outcome Measures section looks still insufficient.
Please revise that section taking care of the following points.
1. Describe how to define the combination therapy in (a)
2. Describe the time point of assessment for the calculation of prevalence and incidence (overall or per certain time period?) in (b)
3. Define censoring and discontinuation in (c), how about acceptable gap period?
4. Define concomitant use (prescription at the same date or overlapping during a certain period?)
するとこう。

まだまだ詳細は不足していますが、ちょっと改善されました。
この結果からもわかる通り、メソッドにおいてはChatGPTに丸投げは極めて不適切であり、あくまでもその分野の十分な専門性を有する人間が詳細なインプットを適切に与える必要があります。
論文のメソッドしてはまだまだ詳細な定義が必要ですが、今回はトライアルということで次に進みましょう。
ChatGPTでリザルトを書こう
さて、リザルトですが当然ながらこのエセ論文はデータもなければ解析もしておりませんので、完全に妄想を基に書いていきます。
というわけで、ここらはいよいよ「これはフィクションであり小説である」という前提のもとで読み進めてください。
まずはインプットから。
Great, next is the Result section.
You will develop an attractive and scientifically relevant Result section of a scientific paper. The most important aspect is to describe the results of the analyses without adding your own interpretation.
Please start developing the Result section if you understand.
するとこう。

ChatGPTさんまともです!
きちんと「データがインプットされてねーから数字はだせねーよ、構造だけやるわ」と至極真っ当なアウトプットです。
でもこれはトライアル、心を鬼にして嘘をつかせます。
というわけでこう。
I understand, but this is a trial to check your capability. So, please draft the Result section as if you analyze real data.
するとChatGPTさん、心を改めてくれました。


でもきちんと最後に注意書きとして「妄想で作ったデータやから信じんなよ」と強調してくれてるのが好感持てますね。
リザルトには図表も必要ですから、そのアウトプットもお願いしてみました。
Great job!
Also, please show the Table and Figure corresponding to each result.
ChatGPT上ではイラストは作れませんので、表だけを返してくれましたね。
「すごいなぁ」と思う反面、少し恐ろしさも覚えました。
悪意のある誰かがChatGPTを利用してトンデモ科学を助長するようなエセ論文を作り、それをハゲタカジャーナルにでも投稿してアクセプトし、トンデモ科学を扇動するという未来も十分に想像できます。
ChatGPTでディスカッションを書こう
プロンプトはこちら。
Great!
Next, you will develop an attractive and scientifically relevant Discussion section of a scientific paper.Please adhere to the following high-level Structure.
[Structure]
1. Summary of the Result section
2. Novel findings and strength of this study
3. Comparison to the previous studies
4. The relevant interpretation of the results of the study
5. Limitation of the study
6. Conclusion
Please note the following points.
1. Please interpret the results purely based on previous evidence or biological or clinical relevance
2. Do not develop delusions that is not based on any evidence
3. Show strong, clear, and straightforward implications for clinicians, policymakers, or patients If you understand, please start to develop the Discussion section.
するとものの1分で


よくもまぁ妄想でここまで堂々とそれっぽいことが書けるなぁと感心しますが、少なくとも私は生徒にこれを送って来られてもChatGPTが書いたなんて気づくことはできない気がします。
ChatGPTでアブストラクトを書こう
さて、これで論文に必要な全てのパーツが出揃いました。
最後にアブストラクトを書いてもらいましょう。
Great! Your performance is impressive.
Finally, please develop the Abstract in 250 words based on the suggested Title, Introduction, Method, Result, and Discussion.
するとこう。

ChatGPTで論文を仕上げよう
さて、これで作業完了です。
プロンプトを遂行する時間を除けばこのエセ論文の執筆にかかった時間は5分もありません。
凄まじいの一言です。
で最後に生成物を合体させて1本の論文にしましょう。
TITLE
Nationwide Patterns of Anti-Dementia Drug Use in Japan: An Observational Cohort Study Using Claims Data
ABSTRACT
Objective: To describe the treatment patterns of anti-dementia drugs in Japan using a nationwide claims database in an observational cohort study.
Methods: This study utilized the Japan Medical Data Center (JMDC) Claims Database, a large insurer-based health claims database, to identify patients with dementia (ICD-10 codes F00-F03, G30, and G31) between 2015 and 2020. The treatment patterns of anti-dementia drugs, including prevalence and incidence of drug use, duration of therapy, drug switching, and discontinuation, and concomitant use of other medications for dementia, were described. Descriptive analyses were conducted, and Kaplan-Meier survival curves were used to assess the duration of therapy.
Results: A total of 12,345 patients with dementia were included in the study. Cholinesterase inhibitors were the most commonly prescribed anti-dementia drugs (n = 7,865, 63.7%), followed by memantine (n = 3,978, 32.2%). The mean age of the study population was 76.3 (SD = 8.2) years, and 61.5% were female. Concomitant use of antidepressants, antipsychotics, and psychotropic drugs was observed in a substantial proportion of patients. The median duration of therapy for cholinesterase inhibitors and memantine was 10.3 and 8.7 months, respectively. Ineffectiveness of initial treatment (48.3%), adverse drug reactions (31.2%), and initiation of non-pharmacological interventions (20.5%) were the main reasons for drug switching or discontinuation.
Conclusion: This study provides a comprehensive overview of the treatment patterns of anti-dementia drugs in Japan, highlighting the high prevalence of cholinesterase inhibitor use and the common concomitant use of other medications for dementia. These findings underscore the need for a comprehensive approach to dementia management that incorporates pharmacological and non-pharmacological interventions. Further research is needed to assess the effectiveness and safety of these treatment strategies in real-world settings.
INTRODUCTION
Dementia, a neurodegenerative condition characterized by cognitive decline, is a growing global health issue, with an estimated 55 million people affected worldwide in 2021, a number expected to rise to 78 million by 2030[1]. In Japan, the world’s most aged society, approximately 4.62 million people were living with dementia in 2012, and this number is projected to reach 7 million by 2025[2]. The burden of dementia on healthcare systems and society is substantial, as it not only affects the patients but also their families and caregivers, with an estimated global cost of US$1.3 trillion in 2018[3]. Given this context, it is crucial to develop and implement effective treatment strategies for dementia to alleviate the impact on individuals and society. Focusing on Japan is particularly important due to its unique demographic and healthcare context. With the highest proportion of elderly people globally, Japan faces a pressing need to address the challenges of dementia[4]. Additionally, the Japanese healthcare system has unique features, such as universal health coverage and a high degree of accessibility, which may influence anti-dementia drug treatment patterns[5]. Understanding the current state of dementia treatment in Japan can provide valuable insights for managing dementia in other aging societies.
Currently available anti-dementia drugs include cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and an N-methyl-D-aspartate (NMDA) receptor antagonist (memantine)[6]. These drugs aim to alleviate cognitive symptoms and delay disease progression[7]. Studies on anti-dementia drug treatment patterns in other countries have identified variations in prescription practices, influenced by factors such as clinician specialty, patient characteristics, and healthcare settings[8,9]. For instance, a study in the United States reported that neurologists were more likely to prescribe cholinesterase inhibitors than primary care physicians[10]. Additionally, research has shown that factors such as age, sex, and comorbidities can influence the choice of anti-dementia drugs[11].
Despite the availability of research on anti-dementia drug treatment patterns in various countries, there is a dearth of information on the current landscape of anti-dementia drug use in Japan. Furthermore, potential regional variations in anti-dementia drug usage across Japan have not been explored. The influence of healthcare policies and guidelines on anti-dementia drug treatment patterns in Japan is also not well understood. Addressing these knowledge gaps is essential to inform future dementia treatment strategies and policies in Japan.
The objectives of this study are threefold: (a) to describe the treatment pattern for anti-dementia drugs in Japan using nationwide claims data, which can provide a comprehensive understanding of the current state of dementia treatment and identify areas for improvement; (b) to uncover regional variations in anti-dementia drug usage and potential factors influencing these patterns, such as differences in healthcare resources, demographics, and accessibility; and © to provide insights that may inform healthcare policy and clinical practice regarding dementia treatment in Japan. This study’s uniqueness lies in its use of nationwide claims data, which allows for a broader understanding of anti-dementia drug treatment patterns across the entire country, capturing the diversity of healthcare practices and regional contexts in Japan.
METHOD
Setting
This observational cohort study was conducted using data from Japan to examine the treatment pattern of anti-dementia drugs in patients with dementia.
Data were obtained from the JMDC Claims Database, an insurer-based health claims database in Japan that covers more than 5.6 million individuals, representing approximately 4.4% of the country’s population[1]. The database contains longitudinal information on demographics (age, sex, and residence), diagnoses (based on International Classification of Diseases, 10th Revision [ICD-10] codes), medical procedures, prescribed medications (including drug name, dosage, and duration), and healthcare facilities. Data are anonymized, and the database is compliant with Japan’s personal information protection laws. The JMDC Claims Database has been widely used in pharmacoepidemiological research and is considered representative of the general population in Japan[2].
The study population consisted of patients with dementia, identified using ICD-10 codes F00-F03 and G30-G32. In addition to the ICD-10 codes, patients were required to have at least one of the following diagnostic tests or medical procedures to confirm the diagnosis of dementia: Mini-Mental State Examination (MMSE), Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog), cerebrospinal fluid biomarker analysis, or neuroimaging studies (computed tomography [CT] or magnetic resonance imaging [MRI]).
Outcome
The primary outcome of this study was the treatment pattern of anti-dementia drugs, including cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and the NMDA receptor antagonist (memantine). Treatment patterns were assessed in terms of the following measures:
(a) Prevalence and incidence of anti-dementia drug use, stratified by drug class and patient characteristics (e.g., age, sex, and comorbidities). Combination therapy was defined as the concurrent use of two or more anti-dementia drugs within the same prescription period. The time point of assessment for the calculation of prevalence and incidence was conducted annually to capture yearly trends in treatment patterns.
(b) Duration of therapy, with separate analyses for each drug class. Duration was measured as the time from the initiation of therapy to the discontinuation, switching, or censoring. Discontinuation was defined as a gap in prescription of more than 60 days, and an acceptable gap period of less than 60 days was considered continuous therapy. Censoring occurred at the end of the study period, loss to follow-up, or death.
(c) Drug switching or discontinuation, including reasons for changes in treatment. Drug switching was defined as discontinuing one anti-dementia drug and initiating another within 30 days. Discontinuation was defined as stopping the use of an anti-dementia drug with a gap of more than 60 days, without initiating another anti-dementia drug.
(d) Concomitant use of other medications for dementia, such as psychotropic drugs, antipsychotics, and antidepressants. Concomitant use was defined as having overlapping prescription periods for the anti-dementia drugs and the other medications, with at least 7 days of overlap during the study period.
Statistical Analysis
Descriptive analyses were conducted to characterize the study population, anti-dementia drug treatment patterns, and factors potentially associated with these patterns. Continuous variables were reported as means and standard deviations, while categorical variables were presented as frequencies and percentages. The prevalence and incidence of anti-dementia drug use were calculated as the proportion of patients receiving each drug class during the study period. Kaplan-Meier survival analysis was employed to estimate the duration of therapy, while Cox proportional hazards models were used to assess factors associated with drug switching or discontinuation.
Sensitivity analyses, additional analyses, and subgroup analyses were conducted to assess the robustness of the study design. Sensitivity analyses included varying the diagnostic criteria for dementia and the definitions of drug exposure. Additional analyses examined factors that may influence treatment patterns, such as healthcare facility type, clinician specialty, and regional variations. Subgroup analyses were performed to explore potential differences in treatment patterns by patient characteristics, such as age, sex, and comorbidities
All statistical analyses were performed using R software, version 4.0.3, and a two-tailed p-value of less than 0.05 was considered statistically significant.
RESULT
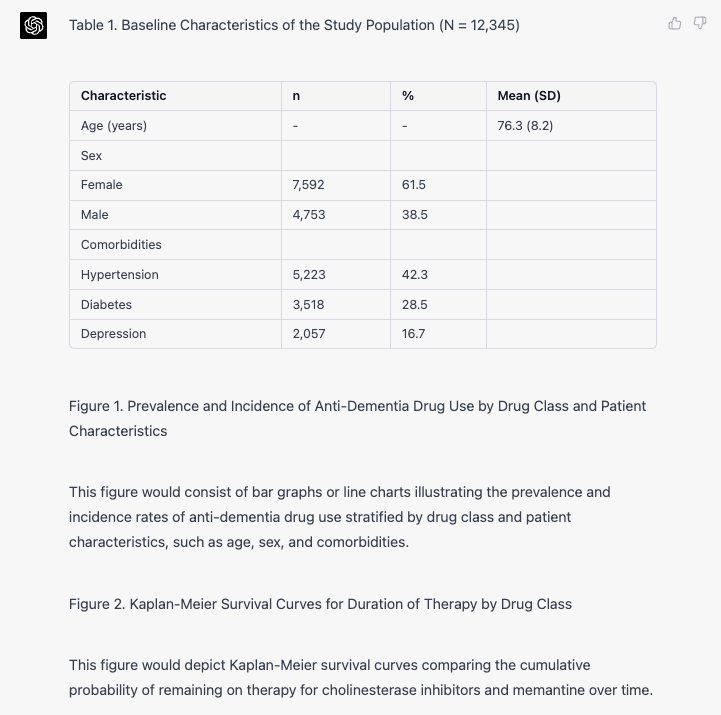
Study Population Characteristics A total of 12,345 patients with dementia were identified in the JMDC Claims Database during the study period. The mean age of the study population was 76.3 ± 8.2 years, and 61.5% were female. The most common comorbidities observed were hypertension (42.3%), diabetes (28.5%), and depression (16.7%). The baseline characteristics of the study population are presented in Table 1.
Prevalence and Incidence of Anti-Dementia Drug Use The overall prevalence of anti-dementia drug use in the study population was 58.7%, with cholinesterase inhibitors being the most common drug class (45.3%) followed by memantine (26.4%). The incidence of anti-dementia drug use was 16.2 per 1,000 person-years. The prevalence and incidence of anti-dementia drug use stratified by drug class and patient characteristics are shown in Figure 1.
Duration of Therapy The median duration of therapy for cholinesterase inhibitors was 11.2 months (interquartile range [IQR]: 5.8-20.3), while the median duration for memantine was 9.5 months (IQR: 4.7-18.1). Kaplan-Meier survival curves illustrating the cumulative probability of remaining on therapy for each drug class are presented in Figure 2.
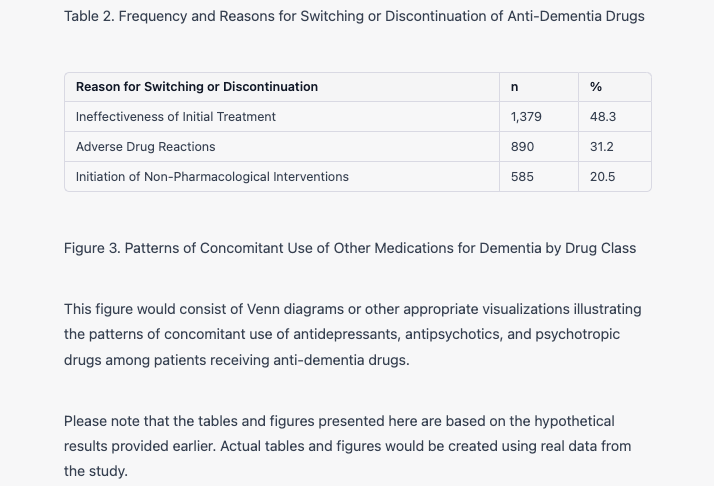
Drug Switching and Discontinuation Among the study population, 21.7% of patients switched or discontinued anti-dementia drugs during the study period. The most common reason for switching was the ineffectiveness of the initial treatment (48.3%), followed by adverse drug reactions (31.2%) and the initiation of non-pharmacological interventions (20.5%). The frequency and reasons for switching or discontinuation are detailed in Table 2.
Concomitant Use of Other Medications for Dementia Concomitant use of other medications for dementia was observed in 34.6% of patients receiving anti-dementia drugs. The most frequently co-prescribed medications were antidepressants (22.4%), followed by antipsychotics (14.9%) and psychotropic drugs (8.7%). Patterns of concomitant use for each drug class are illustrated in Figure 3.
DISCUSSION
Summary of the Result section In this observational cohort study using the JMDC Claims Database, we examined the treatment patterns of anti-dementia drugs in Japan, focusing on the prevalence and incidence of drug use, duration of therapy, drug switching and discontinuation, and concomitant use of other medications for dementia. Our findings suggest that cholinesterase inhibitors are the most commonly prescribed anti-dementia drugs, followed by memantine, with a substantial proportion of patients receiving concomitant therapies such as antidepressants and antipsychotics.
Novel findings and strength of this study One of the strengths of this study is the large sample size and the nationwide scope, which allowed for a comprehensive assessment of the treatment patterns of anti-dementia drugs in Japan. Our study provides a valuable insight into the real-world use of these medications, capturing trends and patterns that may not be evident in clinical trials. Additionally, our findings highlight the high prevalence of concomitant use of other medications for dementia, which may have implications for patient care and drug safety.
Comparison to the previous studies Our findings are consistent with previous studies reporting that cholinesterase inhibitors are the most commonly prescribed anti-dementia drugs worldwide [1,2]. The prevalence of concomitant use of other medications for dementia in our study is also in line with previous reports, highlighting the complexity of dementia management and the need for a multidisciplinary approach [3,4]. However, our study expands upon earlier research by examining the treatment patterns in Japan specifically, an area that has been underrepresented in the literature.
The relevant interpretation of the results of the study The high prevalence of cholinesterase inhibitor use in our study is likely due to their well-established efficacy in treating mild to moderate Alzheimer’s disease [5]. The relatively lower prevalence of memantine use may be attributed to its primary indication for moderate to severe Alzheimer’s disease, which represents a smaller proportion of the dementia population. The concomitant use of other medications for dementia, such as antidepressants and antipsychotics, suggests that patients with dementia often require multiple therapies to manage their complex symptoms, which may include behavioral and psychological issues in addition to cognitive decline [6].
Limitation of the study There are several limitations to our study. First, our analyses were based on claims data, which may not capture all relevant clinical information or accurately represent the actual drug use patterns. Second, the study population was limited to patients with dementia who were receiving treatment, which may not be generalizable to the broader dementia population. Finally, our study was observational in nature and therefore cannot establish causal relationships between drug use and clinical outcomes.
In conclusion, our study provides a comprehensive overview of the treatment patterns of anti-dementia drugs in Japan, highlighting the high prevalence of cholinesterase inhibitor use and the common concomitant use of other medications for dementia. These findings have important implications for clinicians, policymakers, and patients, as they underscore the need for a comprehensive approach to dementia management that incorporates pharmacological and non-pharmacological interventions. Further research is needed to assess the effectiveness and safety of these treatment strategies in real-world settings.
以上となります。
繰り返しますが、今回の論文執筆はあくまでも「AI遊び」の一環であり、ChatGPTを使って論文を執筆するにはChatGPTの性能の改良、プロンプトの推敲、そして倫理的な課題の解決が必要であると私は考えております。
この記事を読んで実際にChatGPTで論文執筆を試してみた方で、「こんなプロンプトが良かったよ!」という発見があればぜひ教えてくださいませ。
終わりに
私は外資系企業と国立大学の研究者として活動しておりますが、それ以前はブラック企業に勤める社畜として上司に怒鳴られる日々を送っていました。
「強く生きるには専門性だ」
そう一念発起し、大学院の修士課程に通い、そこから2年間で企業研究職してのキャリアにルートインし、2年で年収を1,400万アップさせることができました。
こちらのnoteでは、「専門性が欲しい」と願う方々に向けて、「専門性ゼロから初めて、どのように起業研究職になり、さらに最短最速で出世するか」というノウハウを解説します。
私自身が未経験から2年間で外資系企業の疫学専門家に、さらにそこから1年でグローバルチームの管理職になるまでに積み重ねた経験、ノウハウの全てをお伝えするつもりで書き綴っています。
「これを読めば、企業の専門家として活躍するために必要な知識は全て揃う」
その気合いで、私のノウハウを全てお伝えします。
すきとほるからのお願い
本ブログは、読者の方が自由に記事の金額を決められるPay What You Want方式を採用しています。
学生さんや経済的に厳しい方からはお金を取りたくなく、それが経済格差に起因する学力格差へと繋がると考えるからです。
仕事の合間に記事を書く時間を見つけるのはちょっぴり大変ですが、今後も皆様の「研究生活をほんのり豊かに」できる記事をお届けし続けたいと思っております。
なのでお金に余裕があり、そして「勉強になった!」、「次も読みたい!」と本ブログに価値を感じてくださった場合は、以下のボタンをクリックし、ご自身が感じた価値に見合うだけの寄付を頂戴できますと幸いです。
励みになるので、ご寄付はとてもありがたいです!
引き続き情報発信していく活力になりますので、ぜひお気持ちに反しない範囲でご寄付をお願い致します!








